|
|
|
 |
 |
| |
|
|
Home > Teleradiology Services >
Clinical Trials Radiology |
|
|
 |
|
| |
|
|
| |
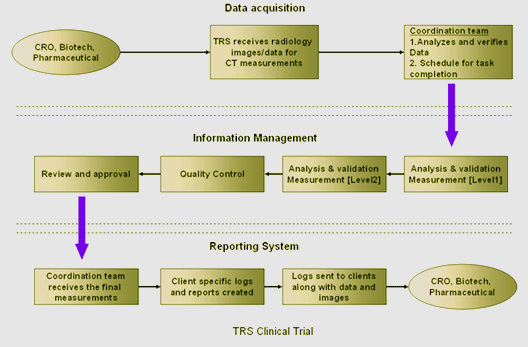
The duration of a drug development cycle can be
significantly prolonged by radiologic reporting
delays. Teleradiology Solutions offers
pharmaceutical vendors, clinical research
organizations, and biotechnology organizations the
opportunity to shorten their development cycles by
using TRS Clinical Trials Radiology Reporting
service
|
|
| |
|
|
| |
|
|
|
 |
| |
|
 |
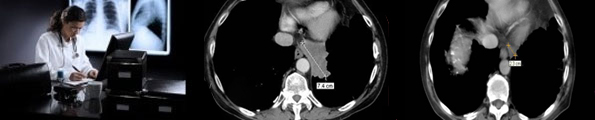
Reporting by radiologists experienced in RECIST and WHO measurement criteria |
|
 |
Rapid and
timely report turnaround |
|
 |
Thorough, accurate and reliable
documentation |
|
 |
Experienced and accessible coordinators to
work with |
|
 |
Reasonable pricing structure |
|
 |
Proven competence in the field |
|
|
|
|
|
|
|
|
Click here for sample
clinical reporting format |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| |
|
|
|
|
|
|
| |
 |
| |
 |
| |
|
|
| |
 |
|
| |
|
|
| |
Teleradiology Solutions has been reading our MRI's for
over a thousand cases and we have yet to find an error.
Their reads are timelier, more detailed and more
comprehensive than what we enjoyed before. They have
helped us push for higher quality care. Their
radiologists have worked with us both clinically and
administratively: on imaging protocols, workflow to
compare prior studies and the like - with the bottom
line being improvement to our patients' care. Any
workflow obstacles are dealt with promptly and
professionally. We are very satisfied and recommend them
wholeheartedly. |
|
| |
|
|
| |
Larry Yalich, M.D.
Multi-Specialty HealthCare, Baltimore, MD |
|
| |
|
|
|